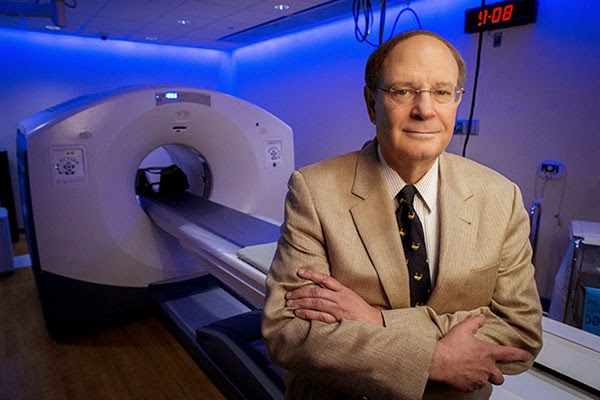
The women’s weight loss is caused by a change in appetite, which results from changes in brain function, explains UAB neuroradiologist Robert Kessler, M.D. (pictured above in UAB's Advanced Imaging Facility). On positron emission tomography (PET) scans, Kessler can see an obvious transformation in the women’s brains, particularly in dopamine neurotransmission.
Using a specialized brain PET scan that he has developed, Kessler can visualize levels of dopamine receptors — molecules that help transmit the brain’s messages of motivation and reward. Before surgery, the women had increased levels of the receptors, which appear on the PET scans as glowing white patches throughout the brain. But after their surgeries, these changes have faded; the women’s brains exhibit a more balanced map of dopamine receptors. In real-world terms, Kessler thinks, these tempered receptor levels reflect a shift to a more normal reward perception, helping the women control their appetites after surgery.
Obesity — and the drive to overeat — isn’t the only pathology that Kessler can see when he peers into people’s brains with a PET scan. During the past 30 years, he has helped illuminate changes to the brain that might underlie schizophrenia, drug addiction, depression and dementia, among other disorders. By looking at a person’s brain PET scan and carefully measuring the levels of neurotransmitter function, Kessler can tell whether someone is more prone to taking risks than average, whether they’re more of a “slacker” or a “go-getter,” and whether or not they have “the ability to experience rewarding stimuli in a normal manner or if they have lost that ability,” he said.
| Find out how a cyclotron works, and what makes UAB’s new cyclotron unique among U.S. academic medical centers, in the video above and in this feature from UAB Magazine. |
“At a very basic scientific level, there’s no other technology that can look at the human brain and inform you about specific molecules and receptors,” Kessler said. At UAB, he’s taking advantage of the university’s TR24 cyclotron — the largest at any U.S. academic medical center — to develop new PET scans. And he has launched collaborations with UAB researchers across the psychiatric and neurological sciences to help them apply his techniques to even more questions.
Focusing on Receptors
As a medical student, resident and fellow in the 1970s, Kessler first became interested in the human brain at a time that clinicians had few methods to visualize the organ. Surgeons could physically see the outer layers of the brain when they opened the skull for an operation, or pathologists could dissect an autopsied brain; but viewing the activity — in a living human — of the molecules that make up the brain’s electrical pathways wasn’t possible.In 1977, Kessler joined a lab at the National Institutes of Health just as this was changing. One of his mentors there became the first to use a PET scan to visualize the activity of the brain. The earliest scans, rather than pinpointing specific receptors as Kessler does now, were designed to simply show which cells in the brain were undergoing metabolism — a sign of activity — at any given moment.
But the basic idea has been the same for more than three decades now: A patient gets an injection of a radioactive tracer into their bloodstream. Depending on the design of the tracer, it accumulates in particular organs or cells of the body. Then, a PET machine is used to measure the location of the accumulated radioactivity.
“It quickly became clear to me that PET was going to become an important tool for understanding the brain,” Kessler said. “And we began to use it to look at everything from brain tumors and schizophrenia to aging and dementia.”
As Kessler immersed himself in the new technology, first at NIH and then at Vanderbilt University, he helped develop new tracers that would pave the way for the rest of his career: 18F-Fallypride, and later 18F-FPEB. Rather than building up in all metabolizing brain cells, these radioactive molecules bind specifically to dopamine and glutamate receptors.
Among the first questions Kessler asked with 18F-Fallypride was ‘What are the effects of antipsychotic drugs on the brains of patients with schizophrenia?’ A new class of antipsychotic drug had recently been developed; the drugs had fewer side-effects than older versions, but researchers didn’t know why. Kessler and his collaborators discovered that the new drugs targeted different areas of the brain than the old drugs, offering not only an explanation for the differences, but a way to test future drugs for their efficacy.
Dopamine had also already become known as a chemical that mediates reward-seeking behavior and pleasure. So Kessler’s lab began to look at how levels of the dopamine receptor and the effects of dopamine release on dopamine receptors might relate to drug abuse, impulse control, addiction and the ability to feel pleasure.
“People who are depressed lose their ability to enjoy rewards and experience the pleasures of life; people who are addicted have very distorted reward functions where they crave just one reward,” Kessler said. “We showed that dopamine plays a key role in all of these.”
 |
| These are 18F-Fallypride PET images of dopamine D2 type receptors, averaged across several normal subjects. There are high levels of these receptors (red color) in deep brain structures and lower levels in the cortex. These include the basal ganglia and thalamus (A), amygdala and temporal cortex (B), and substantia nigra (C). These regions are concerned with movement, emotion and cognition. |
The Future of Brain PET
PET scans offer the most direct way to observe what happens at a molecular level in the brain when someone develops, or recovers from, a psychiatric disorder or addiction, Kessler says. Drug developers and pharmaceutical companies now use PET scans to fine-tune prospective new treatments, he notes. If they know they need to lower the number of dopamine receptors in one area of the brain, for instance, they can use PET scans to determine which drugs, and drug dosages, effectively achieve this.One of the ongoing challenges in the field, Kessler says, is developing new tracers. With UAB’s new cyclotron, Kessler says he has the tools at his fingertips to continue developing and perfecting tracers that bind to different receptors in the brain. He’s already begun work with the neurotransmitter glutamate; like dopamine, glutamate can be studied through PET tracers that bind to glutamate receptors. And glutamate is thought to have roles in autism, Huntington’s disease, Parkinson’s and anxiety disorders, among other things.
This fall, Kessler launched a study examining glutamate receptors in the brains of addicts as they voluntarily withdraw from methamphetamine. The findings — if they show key differences from normal brains — could lead to new drugs to help meth addicts quit their addiction. Studies on Parkinson’s, depression and Alzheimer’s disease are also in the works with UAB collaborators.
Although “this is a tough area to work in for many reasons,” Kessler said — from the vagaries of chemical half-lives to the sheer complexity of the brain itself — he wouldn’t have it any other way. “You just can’t get this kind of information anywhere else.”

No comments:
Post a Comment
We encourage and look forward to your comments on The Mix (and in its related social networking outposts).
Comments will be reviewed before they're posted. Those that are not related to the topic under discussion, promote products or use profanity or abusive language will not be included.